Biosafety Program
Op4.01-2 Biosafety Program
Table of contents
Purpose
Review
Policy and Procedure
IBC
Compliance Officer
Principal Investigators
Oversight & Approval
Training
Disposal
Packaging and Shipping
Consequences of Noncompliance
Approval Process
Purpose
The purpose of the Institutional Biosafety Policy and the Institutional Biosafety Committee (IBC) is to ensure protection of faculty, staff, students who generate, process, and dispose of potentially hazardous biological materials at Missouri State University, as well as others who may become exposed to biological hazards within the university environment. Other areas covered include recombinant DNA research conducted in compliance with the National Institutes of Health Recombinant DNA Guidelines and that protocols of research involving Select Agents (Defined by the Centers of Disease Control and Prevention), including, not limited to recombinant DNA, are reviewed and found to comply with all Federal, State and Local Government requirements. This policy and procedure statement provides for compliance with Federal, State, and Local government regulations concerning biosafety in research and instructional settings.
Review
This policy and procedure statement will be reviewed as needed by the IBC and the Compliance Officer for review and transmission to the Institutional Official.
Policy and procedure
Primary and secondary containment will be accomplished in a manner compliant with the NIH Guidelines for Research Involving Recombinant DNA Molecules (59 FR 34496). This document addresses pertinent research in whole animals and whole plants, as well as in vitro work. Containment is provided by adherence to standard microbiological practices and techniques employing appropriate equipment, in properly designed facilities. Information concerning such practices, techniques, equipment, and facilities at biosafety levels is detailed in Microbiological and Biomedical Laboratories. Proper manipulation of blood and other tissue fluids within the context of “universal precautions” is summarized in Occupational Exposure to Bloodborne Pathogens, Final Rule (29 CFR 1910.1030).
Additionally, registration and management of select agents will be accomplished in accordance with federal law (1996 Anti-Terrorism and the Public Health Security and Bioterrorism Preparedness Act of 2002).
IBC
The IBC is a standing committee that reports to the Institutional Official. It has the responsibility under the NIH Guidelines and on behalf of the University for formulating and recommending biosafety polices and establishing procedures, as well as reviewing research involving matters relating to biosafety for compliance and approving projects judged to be compliant. The committee consists of at least five members appointed by the Institutional Official, including the Director of Environmental Health and Safety and at least two members external to the institution (community members). Membership and qualifications are in compliance with Sections IV-B-2- a and b of the NIH Guidelines. Other ad hoc members may be called upon for specific expertise needs, depending on the research involved (ex., plant experts for plant protocols).
The full IBC will meet at least annually. The full IBC will follow the following procedures:
- The full IBC approves protocols by a majority vote of the membership at the meeting. Although information regarding protocols may be communicated by email, voting must occur at the designated meeting.
- No member of the IBC will vote on a protocol with which he or she as any connection or in which he or she has personal or professional interest other than as a member of the IBC.
- The final result of the voting for each protocol will be recorded, along with any comments and recommendations, and will be communicated to the PI of the protocol. Any recommendations for other committee review (i.e., Institutional Review Board (IRB) or Institutional Animal Care and Use Committee (IACUC) will be communicated by the IBC Chair.
The University has further charged the IBC with responsibility for;
- oversight and establishment of procedures and polices regarding disposal of biohazardous wastes,
- reviewing and advising with regard to situations which represent potential biological hazards, and
- reviewing research personnel, facilities, procedures, and proposals in the area of recombinant DNA technology.
Compliance officer
The compliance officer works closely with the Institutional Official, IBC and the Director of Environmental Health and Safety. The Compliance Officer is generally responsible for implementing university polices and procedures set forth by the IBC, monitoring compliance, reporting problems and violations, assisting laboratory investigators and staff with training of laboratory personnel, and providing technical advice on matters relating to biosafety.
Principal investigators
- Make an initial determination of the required levels of physical and biological containment, and practices and procedures in accordance with NIH Guidelines; determines if the protocol is exempt or requires IBC approval;
- Submits the appropriate paperwork for the purposed work;
- Is responsible for adherence to all requirements of the NIH Guidelines; including required safety practices
- Submits an annual update of the continuing protocols to the IBC;
- Trains all laboratory workers regarding the potential hazards of the work and precautions to be taken;
- Reports any significant problems, or illnesses pertaining to the operation and implementation of containment, or any adverse reactions occurring during studies to the IBC, Compliance Officer and the Director of Environmental Health and Safety;
- Complies with any shipping requirements for DNA molecules;
- Ensures that laboratory workers who work with animals involved in the scope of the work participate in the Occupational Health and Safety Program.
Oversight and approval
Any individual planning to
- use microorganisms, biological toxins, or other materials which may pose a biological hazard for which biosafety level 2 or greater practices, techniques, equipment, or facilities are required, or
- employ recombinant DNA technology must do so with IBC concurrence and approval.
The Director of Environmental Health and Safety should be contacted prior to proposal submission or, in the case of non-funded research prior to study initiation. The Compliance Officer will then assist in presenting the appropriate request to the IBC for review.
All laboratories certified to be biosafety level 2 or above are to be inspected by the Director of Environmental Health and Safety. Individuals planning to obtain materials referenced above which may prose biological hazards for which biosafety level 2 or greater practices, techniques, equipment, or facilities are required must contact the Director of Environmental Health and Safety and the Compliance Officer prior to receipt of such materials.
All work involving DNA must be registered with the IBC and the Office of Environmental Health and Safety. In addition, the following types of DNA experiments must be reviewed and approved by the IBC:
- The deliberate transfer of a drug resistance trait to microorganisms that are not known to acquire the trait naturally, if such acquisition could compromise the drug to control human, animal, or plant diseases,
- The cloning of toxin molecules with LD50 of Less than 100 nanograms per kilogram body weight, and
- The deliberate transfer of DNA into humans.
NOTE: The 3 types of experiments above must also be approved by the NIH and/or OBA (Office of Biotechnology Activities). - The use of Risk Group 2, 3, or 4 restricted organisms as Host vector systems,
- The cloning of DNA from Risk Group 2, 3, or 4 or restricted organisms into non-pathogenic prokaryotic or lower eukaryotic host-vector systems,
- The use of infectious or defective DNA or RNA viruses in the presence of help virus in tissue culture systems,
- The involvement of DNA with whole animals,
- The involvement of DNA with whole plants,
- The involvement of more than 10 liters of culture involving DNA,
- Those not included in the above categories in which all components are derived from on-pathogenic prokaryotes and lower eukaryotes,
- Those involving the formation of DNA molecules consisting of no more than two-thirds of the genome of any eukaryotic virus
- Any work with the CDC Select Agents (PI must be registered with the CDC, as well as receive approval from the IBC).
The following experiments are EXEMPT from the NIH Guidelines and only require registration with the IBC.
- DNA molecules that are not in organisms or viruses,
- Those that consist entirely of a single DNA segment from a single non-chromosomal or viral DNA source, though one or more may be a synthetic equivalent,
- Those that consist entirely of DNA from a single prokaryotic host (including its plasmids or viruses) when propagated only in that host (or closely related strain) or when transferred to another host by well established physiological means,
- Those that consist entirely of DNA segments from a single eukaryotic host (including chloroplasts, mitochondria, or plasmids) when propagated only in that host (or closely related strain);
- Those that consist of DNA segments from different species that exchange DNA by known physiological processes, though one or more of the segments may be a synthetic equivalent (see NIH Guidelines for a list of these exempt exchangers);
- Those that do not present a significant risk to health or the environment (see NIH Guidelines for this list).
No project requiring IBC approval will commence prior to the IBC approval of the protocol.
PIs will be notified annually and asked to complete an annual update form for continuing projects to document that information regarding work procedures, locations, animal use, new personnel training or other significant changes are current with the IBC records. Any problems associated with the work, including violations of guidelines, accidents or illnesses associated with the protocol, or adverse events (in the case of human clinical trials) must be reported to the IBC, and, after review, may require a protocol by the IBC, and/or reporting to the NIH Office of Biotechnology Activities (OBA).
Agricultural field work with transgenic crop species for which USDA-APHIS notification guidelines are applicable does not require IBC approval because it must be performed in a manner compliant with USDA-APHIS guidelines after proposals have been subjected to a permitting process. Researchers should, however, inform the IBC of the proposed research by submitting a copy of the proposal to the Office of Research Administration for filing. The Compliance Officer will forward a copy to the IBC Chair who will notify pertinent members of the intended work. Agricultural field work with all other transgenic plants, invertebrates, and vertebrates, as well as genetically modified microorganisms, must be submitted to the IBC for approval.
Training
It is the responsibility of the principal investigators to provide adequate biosafety training for laboratory personnel under their supervision. The NIH Guidelines stipulate that the principal investigators must have adequate training with regard to good microbiological technique. Furthermore, the principal investigator is responsible for providing laboratory staff with (a) protocols describing potential biohazards and appropriate precautions, (b) training in the area methods to ensure biosafety and address accidents, and (c) information regarding precautionary medical procedures. The Director of Environmental Health and Safety is available to assist laboratory directors with all aspects of such training.
Disposal
Potentially hazardous biological materials are to be considered “regulated waste” and should be disposed of in a manner consistent with federal, state and local government regulations. Contact the Director of Environmental Health and Safety for assistance.
Packaging and shipping
All regulated biohazardous materials will be packaged and shipped in a manner complaint with federal guidelines and codes as they are developed and communicated to the IBC, and the Director of Environmental Health and Safety. Contact the Director of Environmental Health and Safety for assistance.
Consequences of noncompliance
It is imperative that biosafety policies and procedures be strictly adhered to in order to ensure the good health of workers, students and the public and compliance with government guidelines in order not to jeopardize the university with respect to the ability to obtain federal funding. According to the NIH Guide, proposed research which involves special hazards may result in the delay of funding or suspension of work. Grantees and contractors must be prepared to demonstrate that proper standards have been put in place and practice.
Document Last Revised: July 2006
Approval process
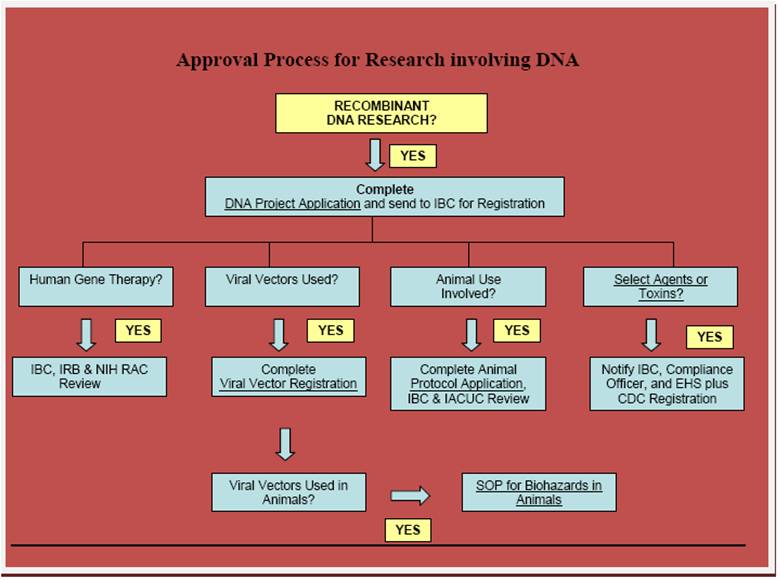
Follow the Branching Scenario of the above flowchart.
Document
Institutional Biosafety Program (MS Word document)*
Institutional Biosafety Program (PDF Document)**
Important :
*You need Microsoft Word to view and print this document.
**You need Adobe Reader to view and print this document.